Get A Quote
BIS Certification for Protective Devices Against Diagnostic Medical X-Radiation – IS/IEC 61331 (Part 3)
With the widespread use of diagnostic
X-ray imaging in medical practices, protecting healthcare professionals and
patients from harmful radiation exposure is of critical importance. Devices
such as lead aprons, thyroid collars, protective eyewear, and patient shields
play a vital role in minimizing the effects of ionizing radiation during diagnostic
procedures.
To ensure these radiation protection products meet stringent safety and performance standards, the Bureau of Indian Standards (BIS) has adopted IS/IEC 61331 (Part 3) — a harmonized Indian and international standard for protective devices against diagnostic medical X-radiation. Let’s explore what this standard entails, why BIS certification is essential, and how manufacturers can obtain it to ensure compliance and safety.
What is IS/IEC 61331 (Part
3)?
IS/IEC 61331 (Part 3) is the Indian
adoption of the International
Electrotechnical Commission (IEC) standard, titled Protective devices
against diagnostic medical X-radiation — Part 3: Protective clothing, eyewear
and protective patient shields.
This standard provides specifications
for materials and construction of
protective devices intended to reduce exposure to X-radiation during diagnostic
procedures such as Fluoroscopy, Radiography, CT scans, and Interventional
procedures. The Indian Standard IS/IEC
61331 (Part 3) outlines comprehensive requirements and test methods for:
1. Protective Clothing: Includes
Lead aprons, Thyroid collars, Gonad shields, Wraparound vests, and skirts.
Must provide radiation attenuation
within specified limits across different kVp (kilovolt peak) energy ranges.
2. Protective Eyewear: Radiation-attenuating
glasses used by radiologists and technicians. Must reduce eye lens exposure to
ionizing radiation. Includes side shielding and optical clarity requirements
3. Patient Shields: Used
to protect sensitive organs (e.g., gonads, breasts, thyroid) during exposure.
Must offer defined levels of attenuation while ensuring comfort and ease of use.
4. Material Testing: All
protective devices must undergo lead
equivalence testing at multiple energy levels, durability assessment (e.g., after bending or repeated use), and visual and mechanical inspection for
defects.
5. Labeling and Marking: Products certified under IS/IEC 61331 (Part 3) must carry the manufacturer’s name and address, lead equivalence value (e.g., 0.25 mmPb, 0.50 mmPb), date of manufacture, and batch number, ISI mark once certified.
Why BIS Certification is
Critical
Medical-grade radiation protection
equipment must adhere to high standards of safety and effectiveness. BIS
certification ensures that products:
● Perform reliably under radiation
exposure
● Protect users and patients as per
defined international norms
● Meet regulatory and hospital procurement standards in India
● Carry the credibility of the ISI mark, boosting trust in healthcare settings
With the implementation of quality control orders (QCOs) across medical device categories, obtaining BIS certification under IS/IEC 61331 (Part 3) is now becoming mandatory for manufacturers of Protective Devices Against Diagnostic Medical X-Radiation.
BIS Certification Process
for IS/IEC 61331 (Part 3)
To obtain BIS certification for
protective X-ray shielding devices, manufacturers must follow these steps:
- Application
Submission: Submit the completed application
form with necessary documentation to BIS.
- Document Review: BIS evaluates the submitted documents to verify accuracy and
completeness.
- Factory Inspection: BIS officials conduct on-site inspections to assess
manufacturing processes and quality control systems.
- Sample Testing: Product samples are collected and tested at BIS-approved
laboratories to verify compliance with Indian standards.
- Certification Grant: Upon successful inspection and testing, the BIS issues the certification, allowing the use of the ISI Mark.
Documents Required for BIS Certification
To apply for ISI Certification for Fire Doors and Assembly, manufacturers must provide the following documents:
● Completed application form
● Detailed manufacturing process information
● Quality control plans and test reports
● Factory layout and equipment details
● Product specifications and technical details
● Business registration proof
● Declaration of conformity to Indian standards
Additional documentation may be required based on product specifications and environmental regulations.
BIS ISI Mark Certification Costing And Timeline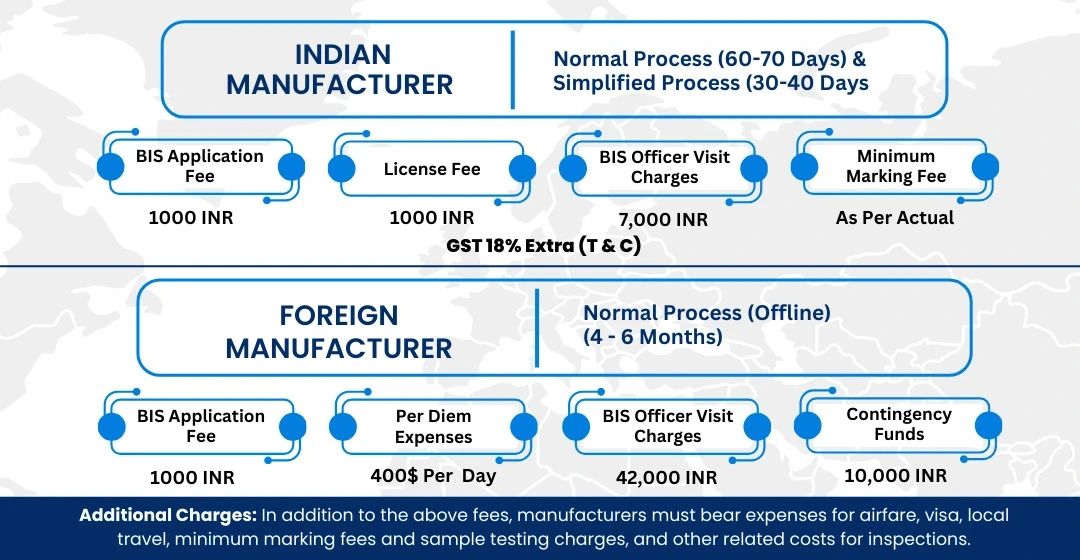
To Know The Process in Detail, Please Visit:
Under BIS Registration Products ISI and CRS
Conclusion
As diagnostic imaging continues to
expand across India, ensuring high-quality radiation protection for healthcare
workers and patients is essential. Adhering to IS/IEC 61331 (Part 3) and
obtaining BIS certification for protective X-ray devices is no longer optional;
it's a vital step toward compliance, safety, and professionalism in the
healthcare ecosystem.
By securing the ISI mark for
protective clothing, eyewear, and patient shields, manufacturers can offer not
only compliance but also peace of mind to users across the medical industry.
Need help with the BIS certification process for radiation protection gear? Our
team can guide you through testing, documentation, audits, and licensing with
expert precision.
Free Call Back
Latest News & Update
📅 BIS Critical Component List (CCL) Updates for Solar PV Modules
🕒 BIS Fee Concessions for MSMEs and Startups | EVTL India
📅 Guidelines for Implementation of Essential Requirements for Security of CCTV
🕒 Omnibus Technical Regulation (OTR) Amendment Order, 2025
🕒 Extension of Timeline for Filing Annual Returns by Battery Producers
📅 Extension of Timeline for Filing Quarterly and Annual Returns for E-Waste
🕒 Extension of Concurrent Running Period for IS 302-1: 2008 and IS 302 (Part 1): 2024
🕒 BIS Guidelines for Grant of Licence (GoL) | EVTL India
📅 CPCB Guidance on filing of Application, Fees and more
🕒 CPCB Notification on Labelling of Plastic Packaging
📅 Mandatory Compliance for Input Materials of Steel and Steel Products for Imports
🕒 BIS Guidelines for Scheme-X Certification for OTR-Regulated Products
📅 BIS Upgrades Product Certification License Numbers to 10-Digit Series
🕒 BIS Certification No Longer Mandatory for 14 Chemical & Polymer Categories
Why Choose EVTL INDIA
Expertise in Indian Regulatory Standards
End-to-End Support
Trusted by Top Indian & Global Brands
Fast Processing & Transparent Pricing
Strong Liaison with Indian Authorities
Company Profile














