Get A Quote
BIS Certification for In Vitro Diagnostic Test Systems for Blood-Glucose Monitoring: IS/ISO 15197

Diabetes is one of the most prevalent
chronic conditions in India, affecting millions of individuals across all age
groups. For effective management, blood-glucose monitoring systems play a vital
role, especially those designed for self-testing by patients. Given the
sensitive nature of these devices and their direct impact on health, their
accuracy, reliability, and safety are non-negotiable.
To ensure standardized quality across
the industry, the Bureau of Indian Standards (BIS) mandates compliance with the
Indian Standard IS/ISO 15197, which lays down stringent requirements for In
Vitro Diagnostic (IVD) Test Systems used in blood-glucose monitoring.
Manufacturers must obtain ISI certification for In Vitro Diagnostic Test
Systems before marketing their products in India.
Let’s discuss the key elements of the Indian Standard for In vitro Diagnostic Test Systems, the importance of the ISI Mark for In vitro Diagnostic Test Systems, and the certification process as per IS/ISO 15197.
What is IS/ISO 15197?
IS/ISO 15197:2013 is the Indian adoption of the international standard ISO 15197. It specifies the performance requirements and evaluation methods for blood-glucose monitoring systems intended for self-testing by individuals with diabetes. The standard covers system accuracy, user performance evaluation, interference testing, information supplied by the manufacturer and labeling and instructions for use. This ensures that the devices not only perform accurately in laboratory settings but also in real-life user environments.
Scope of the Indian
Standard for In Vitro Diagnostic Test Systems
1. Accuracy Requirements: The
standard mandates specific accuracy criteria for glucose meters when compared
to laboratory methods:
2. User Performance Evaluation: Manufacturers must demonstrate that typical users
(non-professionals) can obtain accurate results using the device by following
the instructions.
3. Interference Testing: Devices
must be tested for interference from common substances like medications, oxygen
levels, or hematocrit variation that could affect glucose readings.
4. System Components: The
standard applies to the entire system,
including the meter, test strips, and control solutions, not just individual
parts.
5. Labeling and Instructions: Clear, accurate instructions and labels are required to help users understand device operation, limitations, and interpretation of results.
Why ISI Certification for
In vitro Diagnostic Test Systems is Crucial
Obtaining the ISI certification for In
vitro Diagnostic Test Systems is essential for:
● Ensuring that blood-glucose
monitors are clinically reliable and safe for self-use
● Meeting the regulatory
requirements for sale and distribution in India
● Building consumer trust through
the presence of the ISI Mark
● Qualifying for government
tenders and public health programs
● Supporting better diabetes management outcomes
BIS Certification Process
for IS/ISO 15197
Here’s a step-by-step overview of how
to obtain the ISI Mark for In vitro Diagnostic Test Systems under IS/ISO 15197:
- Application
Submission: Submit the completed application
form with necessary documentation to BIS.
- Document Review: BIS evaluates the submitted documents to verify accuracy and
completeness.
- Factory Inspection: BIS officials conduct on-site inspections to assess
manufacturing processes and quality control systems.
- Sample Testing: Product samples are collected and tested at BIS-approved
laboratories to verify compliance with Indian standards.
- Certification Grant: Upon successful inspection and testing, the BIS issues the certification, allowing the use of the ISI Mark.
Documents Required for BIS Certification
To apply for ISI
Certification for In vitro Diagnostic Test Systems, manufacturers must provide
the following documents:
● Completed application form
● Detailed manufacturing process
information
● Quality control plans and test
reports
● Factory layout and equipment
details
● Product specifications and
technical details
● Business registration proof
● Declaration of conformity to
Indian standards
Additional documentation may be required based on product specifications and environmental regulations.
BIS ISI Mark Certification Costing And Timeline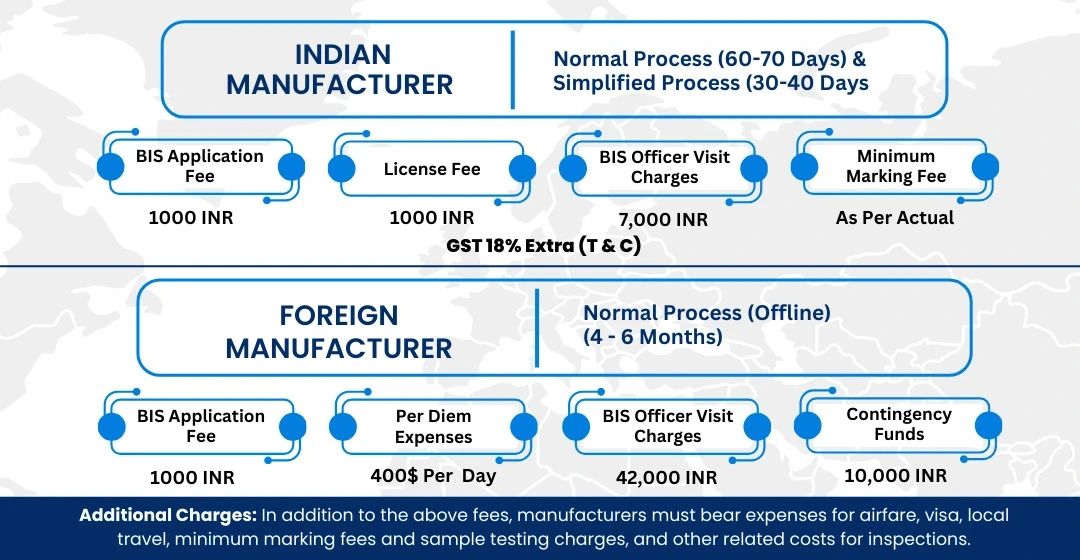
To Know The Process in Detail, Please Visit:
Under BIS Registration Products ISI and CRS
Conclusion
With diabetes on the rise, the role of
accurate and certified blood-glucose monitoring systems is more critical than
ever. By complying with IS/ISO 15197 and obtaining the ISI certification for In
Vitro Diagnostic Test Systems, manufacturers can ensure that their products
meet both clinical standards and regulatory expectations.
The presence of the ISI mark for In
vitro Diagnostic Test Systems gives both healthcare professionals and patients
confidence that they are using a device that is reliable, precise, and tested
under stringent norms. Looking to get your blood-glucose monitoring system BIS
certified? EVTL India experts can guide you through documentation, lab testing,
factory audits, and complete compliance under IS/ISO 15197.
Free Call Back
Latest News & Update
📅 BIS Critical Component List (CCL) Updates for Solar PV Modules
🕒 BIS Fee Concessions for MSMEs and Startups | EVTL India
📅 Guidelines for Implementation of Essential Requirements for Security of CCTV
🕒 Omnibus Technical Regulation (OTR) Amendment Order, 2025
🕒 Extension of Timeline for Filing Annual Returns by Battery Producers
📅 Extension of Timeline for Filing Quarterly and Annual Returns for E-Waste
🕒 Extension of Concurrent Running Period for IS 302-1: 2008 and IS 302 (Part 1): 2024
🕒 BIS Guidelines for Grant of Licence (GoL) | EVTL India
📅 CPCB Guidance on filing of Application, Fees and more
🕒 CPCB Notification on Labelling of Plastic Packaging
📅 Mandatory Compliance for Input Materials of Steel and Steel Products for Imports
🕒 BIS Guidelines for Scheme-X Certification for OTR-Regulated Products
📅 BIS Upgrades Product Certification License Numbers to 10-Digit Series
🕒 BIS Certification No Longer Mandatory for 14 Chemical & Polymer Categories
Why Choose EVTL INDIA
Expertise in Indian Regulatory Standards
End-to-End Support
Trusted by Top Indian & Global Brands
Fast Processing & Transparent Pricing
Strong Liaison with Indian Authorities
Company Profile














