Get A Quote
BIS Certification for Ether IS 336: 1973

BIS Certification
for Ether IS 336:1973 certifies that diethyl ether used in industrial,
laboratory, and pharmaceutical applications meets India's quality and safety
requirements. This certification verifies that the device satisfies the
criteria given in IS 336:1973, ensuring both user safety and performance.
BIS Certification for Ether IS 336:1973 is required for producers and importers seeking legal market access and customer confidence in India. Businesses that achieve compliance demonstrate their dedication to quality assurance, which increases trust in both home and international markets.
Ether is an
important industrial chemical used in medicines and other industries; thus, its
safe manufacture and quality are vital. To guarantee this, the Indian
government enacted the Ether (Quality Control) Order, 2020, which required
producers to comply with IS 336:1973. This standard requires ether to fulfil
particular purity, stability, and labelling standards. Obtaining BIS
Certification for Ether IS 336:1973 is required for authorised sales and
distribution in India. This certification not only validates product quality,
but it also increases market trust and regulatory compliance. To become
certified, manufacturers must adhere to the BIS requirements, testing, and
inspection procedure.
Why is BIS certification necessary for Ether IS 336:1973?
BIS certification
for Ether IS 336:1973 is critical for ensuring the chemical's purity, safety,
and consistency during storage, handling, and industrial applications. Because
ether is highly flammable and can produce explosive peroxides, following IS
336:1973 within the BIS framework ensures compliance with national safety and
purity criteria. This accreditation not only improves product credibility, but
it also allows producers to lawfully sell and distribute ether in India.
Obtaining BIS Certification for Ether IS 336:1973 indicates a company's
dedication to regulatory compliance, customer safety, and environmental
responsibility—all of which are critical for establishing confidence in
sensitive chemical supply chains.
Overview of Indian Standard IS 336:1973
Indian Standard IS 336:1973 applies to ether, a
volatile and flammable organic solvent widely utilised in the pharmaceutical
and chemical sectors. This standard specifies the necessary quality criteria,
testing procedures, labelling standards, and packaging recommendations to
assure the safety, purity, and dependability of ether for commercial
applications. IS 336:1973 guarantees that producers satisfy the Bureau of
Indian Standards' (BIS) regulatory standards, particularly for industrial and
medical purposes. Businesses that produce or import ether must get BIS
Certification for Ether IS 336:1973 to verify product compliance, increase
customer trust, and lawfully sell the material in India under safety and
quality standards.
Process for BIS Certification
The BIS certification process for Ether IS 336:1973 involves
multiple steps designed to thoroughly evaluate a product's compliance with the
required standards. Here is a general overview of the certification process:
1. Application Submission:
Manufacturers must submit an application form along with the required
documentation to BIS.
2. Documentation Review: BIS
reviews the submitted documents to ensure completeness and correctness.
3. Factory Inspection: BIS
officials conduct an on-site inspection of the manufacturing facility to assess
the production process and quality control measures.
4. Sample Testing: Product samples
are taken and tested in BIS-approved laboratories to verify compliance with
Indian standards.
5. Certification Grant: Upon
successful completion of the inspection and testing, BIS grants certification,
allowing the manufacturer to use the BIS mark on their products.
Documents Required for BIS Certification
To apply for BIS certification, manufacturers need to submit the following documents:
● Application form
● Manufacturing process details
● Quality control plan
● Test reports from BIS-approved laboratories
● Factory layout and equipment details
● Proof of business registration
● Product specifications and technical details
● Declaration of conformity to Indian standards
Additionally, manufacturers may be required to provide proof of compliance with environmental and safety regulations, depending on the specific type of product being certified.
BIS ISI Mark Certification Costing And Timeline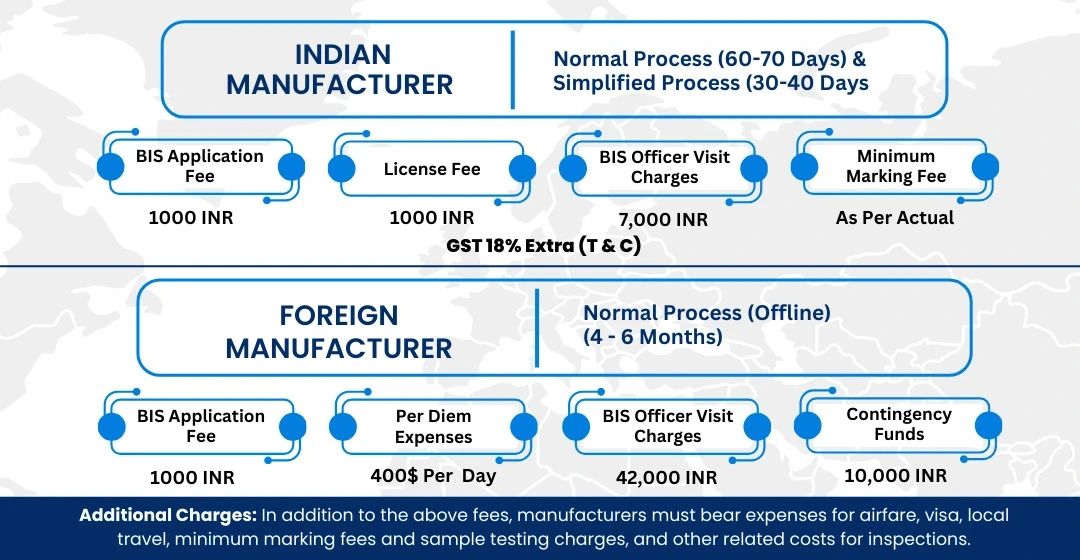
To Know The Process in Detail, Please Visit:
Under BIS Registration Products ISI and CRS
Conclusion
Finally, BIS
Certification for Ether IS 336:1973 is required for producers and importers
working with diethyl ether in India. This certification assures that the
product fulfils the Bureau of Indian criteria's safety, purity, and labelling,
hence ensuring customer safety and product reliability. For enterprises looking
for a simplified certification procedure, EVTL India provides experienced
assistance and end-to-end consultation services for BIS Certification for Ether
IS 336:1973. Our staff assists with everything from documentation to product
testing and communication with BIS authorities, ensuring fast compliance and
clearance. Partnering with EVTL India not only lowers delays and regulatory
concerns but it also increases your product's credibility in the Indian market.
By obtaining BIS Certification for Ether IS 336:1973 with our expertise, you
can position your brand for long-term growth and regulatory certainty. Let us
guide you through the complexity of the certification environment with
confidence and speed.
Free Call Back
Latest News & Update
📅 BIS Critical Component List (CCL) Updates for Solar PV Modules
🕒 BIS Fee Concessions for MSMEs and Startups | EVTL India
📅 Guidelines for Implementation of Essential Requirements for Security of CCTV
🕒 Omnibus Technical Regulation (OTR) Amendment Order, 2025
🕒 Extension of Timeline for Filing Annual Returns by Battery Producers
📅 Extension of Timeline for Filing Quarterly and Annual Returns for E-Waste
🕒 Extension of Concurrent Running Period for IS 302-1: 2008 and IS 302 (Part 1): 2024
🕒 BIS Guidelines for Grant of Licence (GoL) | EVTL India
📅 CPCB Guidance on filing of Application, Fees and more
🕒 CPCB Notification on Labelling of Plastic Packaging
📅 Mandatory Compliance for Input Materials of Steel and Steel Products for Imports
🕒 BIS Guidelines for Scheme-X Certification for OTR-Regulated Products
📅 BIS Upgrades Product Certification License Numbers to 10-Digit Series
🕒 BIS Certification No Longer Mandatory for 14 Chemical & Polymer Categories
Why Choose EVTL INDIA
Expertise in Indian Regulatory Standards
End-to-End Support
Trusted by Top Indian & Global Brands
Fast Processing & Transparent Pricing
Strong Liaison with Indian Authorities
Company Profile














