Get A Quote
BIS Certification for Crepe Bandage IS 4605:2020

In the healthcare industry, product
quality is not just a requirement—it’s a necessity. Among essential medical
textiles, crepe bandages play a vital role in wound care, injury recovery, and
surgical support. To ensure their safety, durability, and effectiveness, the
Bureau of Indian Standards (BIS) mandates certification under the Indian
Standard IS 4605:2020. This blog explores the importance, process, and benefits
of BIS certification for Crepe Bandage.
What Is BIS Certification for Crepe Bandage?
BIS certification for Crepe Bandage
falls under the category of medical textile products, which also includes sanitary
napkins, surgical dressings, infant diapers, and more. These products come into
direct contact with the human body, making it crucial for them to meet rigorous
safety and hygiene standards.
The Indian Standard IS 4605:2020 outlines the specifications for crepe bandages in terms of material, elasticity, absorbency, strength, and biocompatibility. A BIS license ensures the product adheres to these quality norms.
Why Do You Need BIS
Certification for Crepe Bandage?
The manufacturers of crepe bandage
must obtain the ISI mark certification for crepe bandages. It assures
consumers, healthcare providers, and regulatory bodies that the product is
tested in BIS-accredited labs, compliant with Indian quality standards, and
safe for medical use. This certification is more than just a stamp—it's a
promise of performance, hygiene, and care.
● Safety First: Medical textiles must be
free from toxic chemicals and skin irritants. BIS certification guarantees
this.
● Compliance with Standards: It ensures
the product aligns with the Indian
Standard for Crepe Bandage, reducing health risks.
● Consumer Trust: The ISI Mark on packaging signals reliability and government-backed
quality assurance.
● Legal Protection: BIS-certified products
are compliant with regulatory requirements, protecting businesses from legal
issues.
● Market Acceptance: Government tenders and hospitals often mandate ISI certification for procurement.
Process for Applying for
BIS Certification
There are two main pathways for
obtaining BIS certification for Crepe Bandage:
- Application
Submission: Submit the completed application
form with necessary documentation to BIS.
- Document Review: BIS evaluates the submitted documents to verify accuracy and
completeness.
- Factory Inspection: BIS officials conduct on-site inspections to assess
manufacturing processes and quality control systems.
- Sample Testing: Product samples are collected and tested at BIS-approved
laboratories to verify compliance with Indian standards.
- Certification Grant: Upon successful inspection and testing, the BIS issues the certification, allowing the use of the ISI Mark.
Documents Required for BIS Certification
To apply for ISI
Certification for Crepe Bandage, manufacturers must provide the following
documents:
● Completed application form
● Detailed manufacturing process
information
● Quality control plans and test
reports
● Factory layout and equipment
details
● Product specifications and
technical details
● Business registration proof
● Declaration of conformity to
Indian standards
Additional documentation may be required based on product specifications and environmental regulations.
BIS ISI Mark Certification Costing And Timeline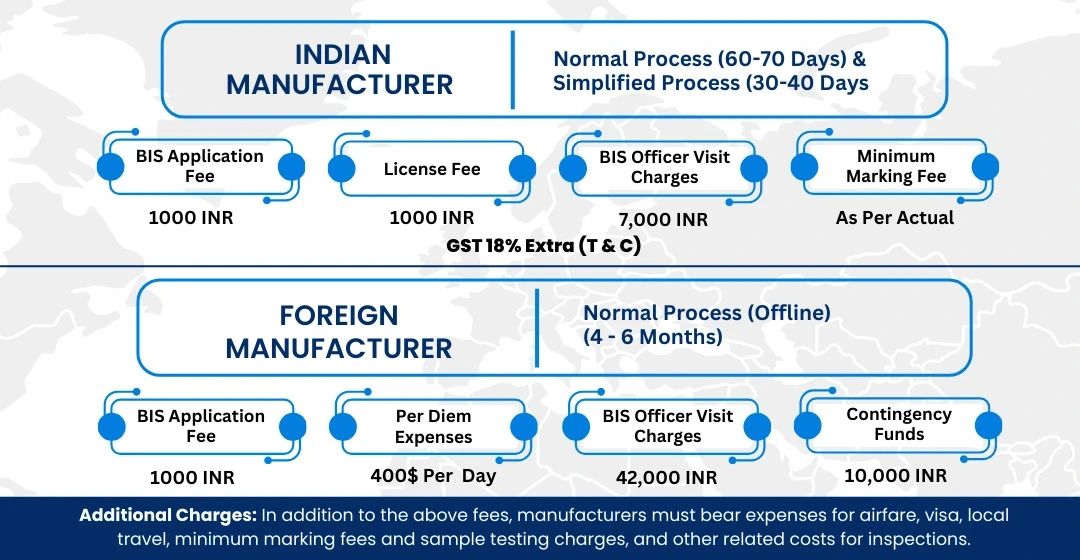
To Know The Process in Detail, Please Visit:
Under BIS Registration Products ISI and CRS
Timeline for BIS
Certification
● Normal Procedure: 40–45 days
● Simplified Procedure: 30–35 days
(quicker due to pre-testing)
The simplified route is often preferred due to its efficiency.
Validity and Renewal of
BIS License
The BIS certification for Crepe Bandage is initially valid for 1 year and can be renewed for up to 5 years. Renewal must be completed within 90 days of expiry. If missed, the applicant must go through the full process again, including re-application and testing.
Conclusion
In the world of medical care, quality
can never be compromised. Crepe bandages, used for support, compression, and
dressing, are essential tools in healthcare. Obtaining BIS certification for
Crepe Bandage is not only a regulatory requirement but a declaration of trust,
quality, and patient safety.
Manufacturers who prioritize certification demonstrate their commitment to delivering safe, high-standard medical products. The ISI Mark is more than a label—it’s your brand’s passport to credibility in a safety-conscious market. Need help applying for BIS certification or navigating compliance for your medical textile products? Feel free to reach out—I'm here to make the complex, simple
Free Call Back
Latest News & Update
📅 BIS Critical Component List (CCL) Updates for Solar PV Modules
🕒 BIS Fee Concessions for MSMEs and Startups | EVTL India
📅 Guidelines for Implementation of Essential Requirements for Security of CCTV
🕒 Omnibus Technical Regulation (OTR) Amendment Order, 2025
🕒 Extension of Timeline for Filing Annual Returns by Battery Producers
📅 Extension of Timeline for Filing Quarterly and Annual Returns for E-Waste
🕒 Extension of Concurrent Running Period for IS 302-1: 2008 and IS 302 (Part 1): 2024
🕒 BIS Guidelines for Grant of Licence (GoL) | EVTL India
📅 CPCB Guidance on filing of Application, Fees and more
🕒 CPCB Notification on Labelling of Plastic Packaging
📅 Mandatory Compliance for Input Materials of Steel and Steel Products for Imports
🕒 BIS Guidelines for Scheme-X Certification for OTR-Regulated Products
📅 BIS Upgrades Product Certification License Numbers to 10-Digit Series
🕒 BIS Certification No Longer Mandatory for 14 Chemical & Polymer Categories
Why Choose EVTL INDIA
Expertise in Indian Regulatory Standards
End-to-End Support
Trusted by Top Indian & Global Brands
Fast Processing & Transparent Pricing
Strong Liaison with Indian Authorities
Company Profile














